Researcher investigates the forces behind complex fluids

Sunscreen, laundry detergent, the can of paint you just picked up from the hardware store. What if we told you that your everyday liquid consumer products aren't just liquids—they're a complex blend of liquid and solid particles?
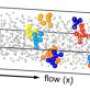
If you were to look very closely—say, with a high-powered microscope—you'd see tiny solid particles suspended in these liquids that are not actually dissolved, but floating in place. The forces of physics keep these particles in place to make your products work. These forces may be microscopic, but they're no small challenge to engineer.
Bob Tilton, professor of chemical and biomedical engineering and director of the Center for Complex Fluids Engineering at Carnegie Mellon University, has received a three-year, $365,000 NSF grant for a project titled "Synergistic or antagonistic effects of polymer/surfactant supramolecular assembly on the colloidal depletion force," which will explore the forces that cause particles to suspend or aggregate in complex fluids.
But what makes certain fluids "complex"?
"Lots of liquids are what we would call simple liquids—everything in the liquid is dissolved," explains Tilton. "We also have liquids that we refer to as complex fluids. There are many different kinds of complex fluids, one of which would be where some of the components of the liquid are not actually dissolved, but are suspended as very small, sub-micron particles."
Take your paint can, for example: the pigment particles used to tint the paint are not actually dissolved. They're solids suspended in a liquid base, whereas dishwashing detergent molecules are truly dissolved, surfactants, that spontaneously self-assemble into bigger swarms of molecules called micelles.
The applications of complex fluids engineering are ubiquitous and significant—the same principles that allow engineers to suspend particles in sunscreen and lotion can be manipulated to planarize, or polish, microchips. Chips wouldn't work if they couldn't be planarized, which is done with suspensions of solid particles in metal polishing liquids. The grit and debris that come off of planarized circuits have to be suspended in the liquid and not redeposited on the surface; the same physical chemistry used to create sunscreen helps in the fabrication of functioning microcircuitry.
Forces between suspended particles are either repulsive or attractive—particles either avoid each other or pull toward each other—and in order to get a stable suspension of particles, engineers need to strike the right balance between those forces. Tilton's project examines how to gain control over "depletion force," a force determined by how many dissolved polymers are able to fit between suspended particles. The smaller the gap between suspended particles, the fewer dissolved polymers can fit between them, and the greater the pressure around the suspended particles, pushing them together. In other words, a higher depletion force means greater attraction.
For a product like paint, a higher depletion force would mean a more watery liquid, as the attracted and clustered pigment particles would sink to the bottom of the can. But if removing particles from a liquid were your goal—like in water treatment processing, for example—then a higher depletion force causing unwanted particles to attract and aggregate out of the solution would be very desirable.
"The thing about the field of complex fluids that I think appeals to so many of us is that the same physical and chemical ideas work in many, many different settings," says Tilton. "It's an enabling discipline for a huge variety of industrial and consumer products, pharmaceuticals, natural environmental processes, and technological applications."