Researchers reveal fail-safe structure of enzyme linked to Alzheimer's, cancer
Like millions of Americans, Harvard Medical School postdoctoral fellow Tom Seegar struggled as he watched several family members decline from Alzheimer's disease.
"Seeing them start to lose what we most value—our minds and ability to think—was especially painful," he said.
Seegar's desire to make a difference for people with Alzheimer's helped motivate him during a five-year project to better understand a molecule linked to the disease—and lends extra meaning to what he discovered.
Working with colleagues at HMS and around the world, Seegar has revealed the atomic structure of ADAM10, a molecule that plays a critical role in healthy cell-to-cell communication but whose malfunction has also been implicated in neurodegeneration, some breast cancers and asthma.
The team's findings, published Dec. 7 in Cell, describe a fail-safe mechanism that prevents the scissor-like ADAM10 from cutting proteins with abandon. ADAM10's structure had a surprise in store as well.
Seeing the detailed shape of ADAM10 deepens researchers' understanding of how the molecule works normally and provides a foundation for probing how it goes awry.
"We're showing researchers what ADAM10 actually looks like," said Seegar, who works in the lab of Stephen Blacklow and is first author of the study. "We need to appreciate its atomic structure to understand how it does its normal job and how it goes down a path toward dysfunction."
The discovery sets the stage for the development of drugs that act specifically on ADAM10 to treat the diseases it fuels.
"Sometimes there's a lag between basic research and its applications to human health, but if we don't have a deep understanding of how fundamental biomolecules work, we're shooting in the dark when it comes to designing therapies," said Blacklow, the study's senior author and the Gustavus Adolphus Pfeiffer Professor and chair of the Department of Biological Chemistry and Molecular Pharmacology at HMS.
The ADAMs family
ADAM10 is just one of 22 ADAMs made by the human body. Short for "a disintegrin and metalloproteinase," these enzymes typically stick out of cell membranes and help cells respond to their environment by snipping away key proteins on the cell surface.
For instance, when a Notch receptor on the surface of a cell receives a message from a neighboring cell, ADAM10 cuts Notch's tether, initiating a chain of events that allows the message to pass into the receiving cell.
In the brain, ADAM10 also does some fancy knife work to process a molecule called amyloid precursor protein, or APP. APP is most famous for being the source of amyloid beta, the plaque-forming substance believed to drive Alzheimer's disease. But APP turns toxic only when processed by beta and gamma secretase enzymes. When certain ADAM family members known as alpha secretases do the job, including ADAM10, the resulting product doesn't form amyloid and appears to be harmless—or even protective.
Given these and other roles in health and disease, researchers have been trying to find out: How exactly does ADAM10 interact with various critical molecules? What prevents it from cutting every protein in sight? Could a drug be designed to manipulate ADAM10 without affecting other ADAMs or similar enzymes? Could ADAM10 be boosted only in the brain or only in the presence of APP, to promote nontoxic APP processing while keeping ADAM10's other activities, like Notch signaling, at normal levels?
By capturing an image of ADAM10, Seegar and colleagues have made it easier for scientists to tackle these questions.
Worth 1,000 words
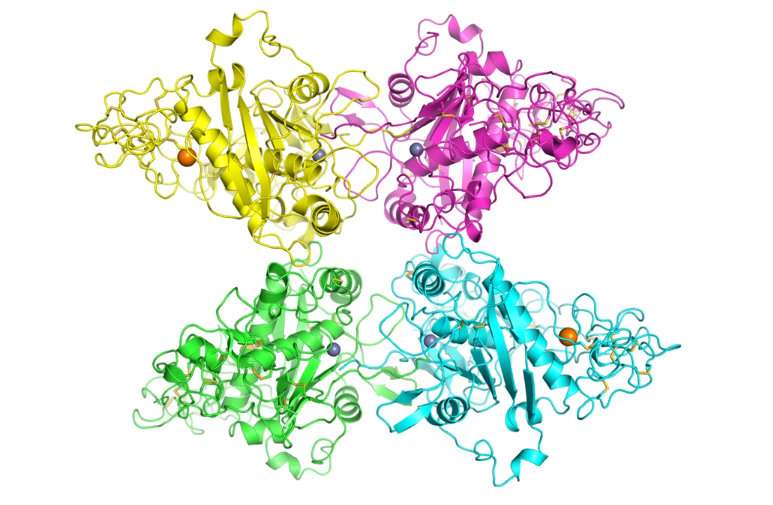
ADAM10 defied expectations when it was finally caught on camera—or rather, on X-ray crystallography.
"It didn't look like what we expected based on ADAM22," the only other ADAM family member whose structure scientists have deciphered, said Blacklow. Nor did it resemble its closest non-ADAM cousins, such as an enzyme in snake venom. It didn't match any predictive models, either.
"An important lesson here is that predictions without experiments can sometimes misdirect you," said Blacklow, "even in an age when our predictive power is becoming ever more compelling."
Whereas ADAM22 looks like a ball and cup, ADAM10 evokes more of an arrowhead shape, according to Seegar, or a seahorse, according to Blacklow.
The team's critical finding revealed that, after young ADAM10 has a little bit cut off from its leading edge to create its mature form, it doesn't just wave around unchecked, "giving every protein on the cell surface a haircut," said Blacklow. Instead, a so-called cysteine-rich domain covers the pocket containing ADAM10's eager blades, unsheathing the scissors only when the right stimulus arrives.
"It maintains ADAM10 in a restrained, or inactive, state," said Seegar. "We weren't the first to hypothesize such a fail-safe, but we were the first to show it."
In essence, the team demonstrated that ADAM10 has a "business end" kept in check by a "regulatory end," said Blacklow.
Next, the researchers want to find out how the pocket gets revealed and how ADAM10 recognizes APP, among other questions.
In the meantime, they've made ADAM10's structural details publicly available, "so anyone who wants to develop treatments for Alzheimer's can access it," said Blacklow.
More information: Tom C.M. Seegar et al. Structural Basis for Regulated Proteolysis by the α-Secretase ADAM10, Cell (2017). DOI: 10.1016/j.cell.2017.11.014
Journal information: Cell
Provided by Harvard Medical School