Scientists create predictive model for hydrogen-nanovoid interaction in metals
A five-year collaborative study by Chinese and Canadian scientists has produced a theoretical model via computer simulation to predict properties of hydrogen nanobubbles in metal.
The international team was composed of Chinese scientists from the Institute of Solid State Physics of the Hefei Institute of Physical Science along with their Canadian partners from McGill University. The results will be published in Nature Materials on July 15.
The researchers believe their study may enable quantitative understanding and evaluation of hydrogen-induced damage in hydrogen-rich environments such as fusion reactor cores.
Hydrogen, the most abundant element in the known universe, is a highly anticipated fuel for fusion reactions and thus an important focus of study.
In certain hydrogen-enriched environments, e.g., tungsten armor in the core of a fusion reactor, metallic material may be seriously and irreparably damaged by extensive exposure to hydrogen.
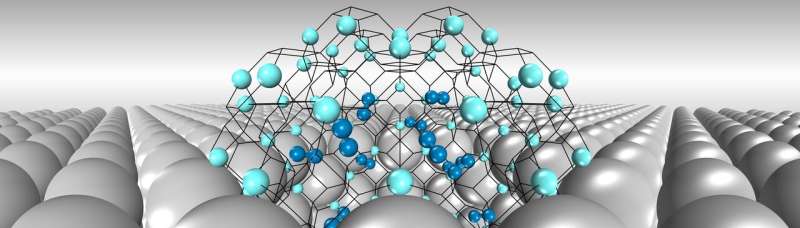
Being the smallest element, hydrogen can easily penetrate metal surfaces through gaps between metal atoms. These hydrogen atoms can be readily trapped inside nanoscale voids ("nanovoids") in metals created either during manufacturing or by neutron irradiation in the fusion reactor. These nanobubbles get bigger and bigger under internal hydrogen pressure and finally lead to metal failure.
Not surprisingly, the interplay between hydrogen and nanovoids that promote the formation and growth of bubbles is considered the key to such failure. Yet, the basic properties of hydrogen nanobubbles, such as their number and the strength of the hydrogen entrapped in the bubbles, has largely been unknown.
Furthermore, available experimental techniques make it practically impossible to directly observe nanoscale hydrogen bubbles.
To tackle this problem, the research team proposed instead using computer simulations based on fundamental quantum mechanics. However, the structural complexity of hydrogen nanobubbles made numerical simulation extremely complicated. As a result, the researchers needed five years to produce enough computer simulations to answer their questions.
In the end, however, they discovered that hydrogen trapping behavior in nanovoids—although apparently complicated—actually follows simple rules.
First, individual hydrogen atoms are adsorbed, in a mutually exclusive way, by the inner surface of nanovoids with distinct energy levels. Second, after a period of surface adsorption, hydrogen is pushed—due to limited space—to the nanovoid core where molecular hydrogen gas then accumulates.
Following these rules, the team created a model that accurately predicts properties of hydrogen nanobubbles and accords well with recent experimental observations.
Just as hydrogen fills nanovoids in metals, this research fills a long-standing void in understanding how hydrogen nanobubbles form in metals. The model provides a powerful tool for evaluating hydrogen-induced damage in fusion reactors, thus paving the way for harvesting fusion energy in the future.
More information: Predictive model of hydrogen trapping and bubbling in nanovoids in bcc metals, Nature Materials (2019). DOI: 10.1038/s41563-019-0422-4
Journal information: Nature Materials
Provided by Chinese Academy of Sciences