February 28, 2020 feature
An understudied cause of cancer: Mutations in regulators of cell signaling
Mutations in a vital class of regulatory molecules are an underappreciated cause of cancer because they impair the function of "G" proteins, a versatile and vast family of signaling switches that underlie innumerable biological facets of life itself.
G proteins are so ubiquitous they are said to be involved in everything from sex in yeasts to cognition in humans, report a team of investigators at Boston University.
Indeed, thick biology textbooks include extensive chapters on G-protein signaling and how these vital extra- and intracellular messages are relayed through receptors known as G-protein-coupled receptors. The letter G stands for the compound guanine.
With the Boston scientists' discovery of mutations in a vital group of signaling regulators, the team has begun to shed new light on a previously elusive cause of cancer development. Relying on several methodologies to study the mutations, the researchers, led by Drs. Mikel Garcia-Marcos and Vincent DiGiacomo, linked the genomic flaws to numerous tumor types, including lung, brain and malignant skin cancers.
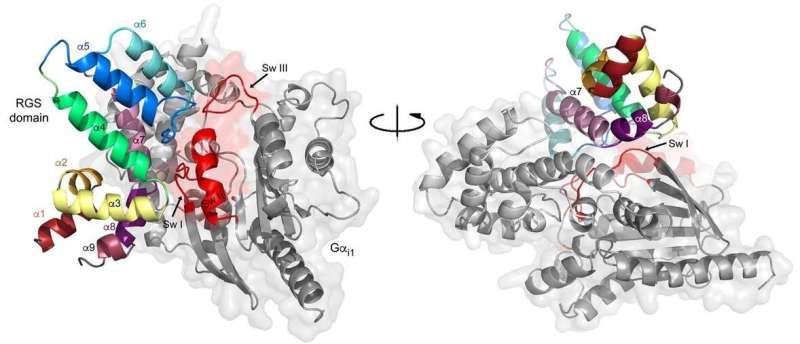
Their most prominent finding was genetic miscues in a key protein—the regulator of G-proteins, a molecule abbreviated as the RGS protein, a master regulator of G signaling. These molecules normally restrain G protein signaling through a phenomenon called GAP activity that relies on a molecular domain known as the RGS-box.
The researchers created a method to study cancer-linked mutations in 20 related RGS proteins. All told, 475 mutations were found in 22 types of cancer. Nine of the mutations interfered with RGS proteins in human kidney cells alone, the scientists found.
In their study, the team devised an "experimental pipeline" to systematically assess a "mutational landscape" in an analysis that allowed them to identify hundreds of low-frequency mutations.
Trimeric G proteins relay signals from the vast superfamily of G-protein-coupled receptors, or quite simply, GPCRs. G proteins can switch on a cascade of signaling events that culminate in a change in cell function. G proteins and GPCRs, when functioning together, transmit signals from hormones, neurotransmitters and a host of small molecules. Trimeric means that a G protein has three segments: alpha, beta and gamma.
"The main advance provided by this work is to fill a gap between already available genomic data and the lack of knowledge about the associated functional consequences for a family of important regulators," Garcia-Marcos and DiGiacomo wrote in the journal Science Signaling.
By way of bioinformatics analysis, genetic interaction studies in yeast, and functional assays in mammalian cells, the team demonstrated that many cancer-associated mutations failed to inhibit G protein signaling because of reduced protein stability or impaired interactions with their targets.
Together, G proteins and GPCRs are involved in myriad signaling activities that underlie the many vital forces of life. To appreciate that fact is to step into a molecular world of a vast and complex communications network.
GPCRs form the largest family—indeed a superfamily—of cell surface receptors common among eukaryotes. The GPCRs are in charge of mediating an organism's response to signals that are received from the outside world through what is seen, felt and smelled. GPCRs also receive signals from other cells and from chemical mediators, such as hormones and neurotransmitters. GPCRs, some biologists say, are among the most intriguing receptors on the planet.
Humans' sense of sight, smell and taste rely on GPCRs. The GPCR superfamily includes rhodopsin, the light-activated protein in the eye. Biologists estimate that there are more than 700 GPCRs in humans. But mice have about 1,000 GPCRs involved with the sense of smell alone. A remarkable finding about GPCRs in humans is that half of all known pharmaceutical drugs act through GPCRs or the signaling pathways they activate. Because of their pervasiveness, there should be little surprise that mutations in their RGS regulator can set the stage for cancer.
"Although efforts during the past decade have shed light onto the relevance of GPCR-G protein signaling in human cancer, the role of RGS proteins in this context remains understudied," the Boston scientists said.
More information: Vincent DiGiacomo et al. Probing the mutational landscape of regulators of G protein signaling proteins in cancer, Science Signaling (2020). DOI: 10.1126/scisignal.aax8620
© 2020 Science X Network